Hours, not days
We are improving sepsis treatment and safeguarding the effectiveness of antibiotics for generations to come. ASTar® is our CE-IVD marked rapid AST system, fully automated and delivering results directly from positive blood cultures.
ASTar in the lab ASTar in the clinic50
Million
50 million global cases of sepsis every year. 11 million sepsis-related deaths
20%
Global deaths
Sepsis accounts for 20% of global deaths every year
~½
Children
~½ of sepsis cases are children under the age of five

Get in touch
Contact our team to learn more about ASTar
Sepsis is a life-threatening condition that can affect anyone. Approximately 50 million people are affected every year. 11 million die. Many survivors pay a high price. Shahrzad is one of them.

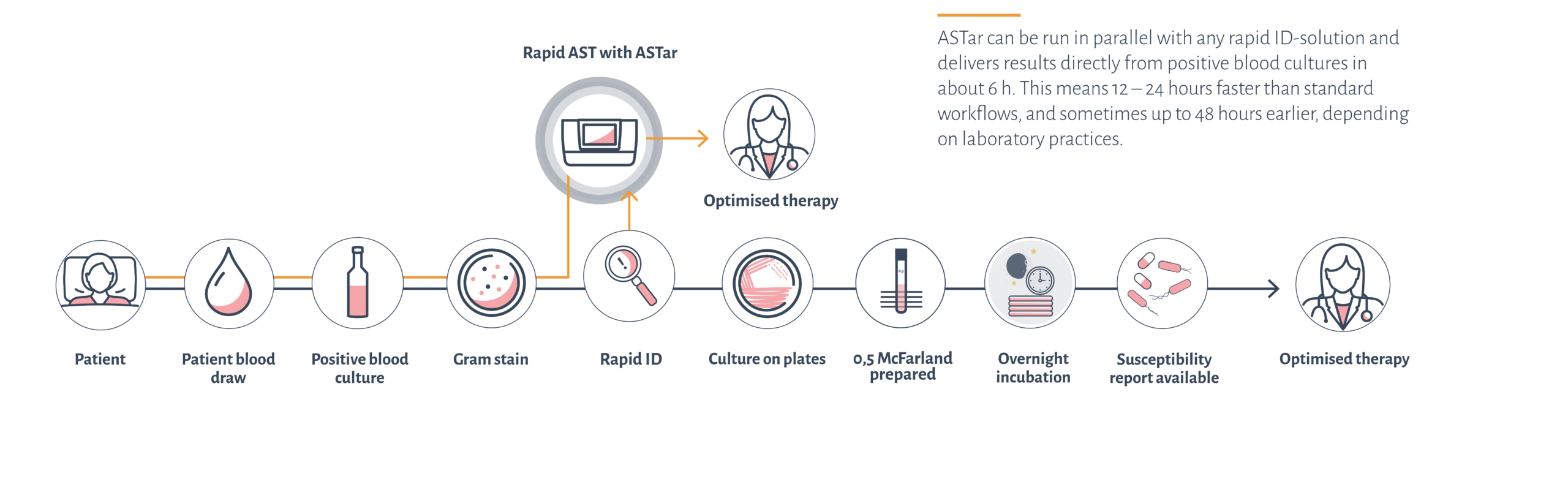
Sepsis and bloodstream infections are time-critical conditions where every hour of delayed appropriate treatment severely affects patient survival. Antimicrobial susceptibility testing (AST) is crucial to determine the best treatment for these patients.
Traditional AST can take upwards of 48 hours. Rapid AST systems are essential for accelerating the diagnostic workflow and treatment decisions.
ASTar is part of the solution.
Experience the difference
Why choose ASTar?

“The ASTar system represents an exciting innovative platform”
Stephen P Kidd, Lead Healthcare Scientist, PhD, Hampshire Hospitals NHS Foundation Trust, presented results from a study in Pro-Lab Diagnostics’ booth at the IBMS 2023 Congress in Birmingham, UK.
“A huge advantage”
Listen to Ehsan Ghaderi, Head of Department, and Sofia Persson, Consultant Physician, Bacteriology Uppsala University Hospital, talk about how ASTar have helped save time in the lab.
Q-linea as an investment
Q-linea is an innovative infection diagnostics company whose ambition is to offer products that benefit patients, healthcare providers and society.
The story of Q-linea
Back in 2008, three people crammed into an office the size of a cleaning closet were working on an idea. That idea led to a international team with a single focus – to improve sepsis treatment.
Want to learn more about ASTar?
Click here