Take action with same-shift AST results
Take action with same-shift AST results
Hours, not days.
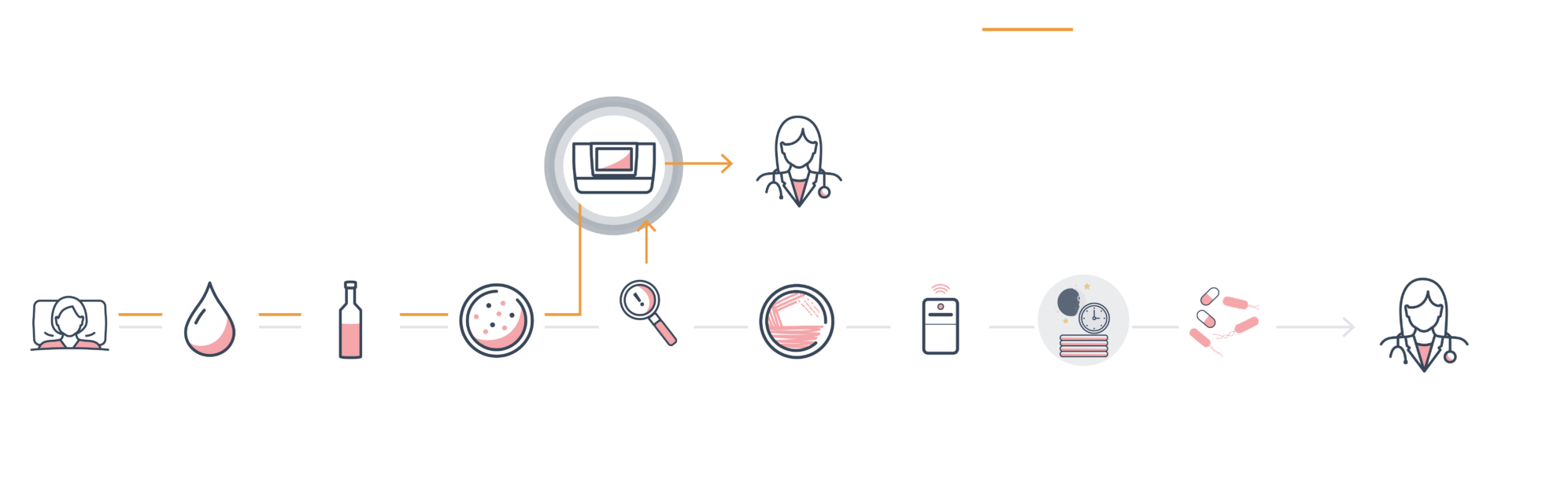
ASTar is a rapid AST system that delivers actionable AST reports in approximately six hours. This is 12–24 hours faster than standard workflows, and sometimes up to 48 hours earlier, depending on hospital routines.
- Adjust broad-spectrum empiric therapies faster.
- Administer appropriate antimicrobial therapies in the same shift from blood culture positivity.
Tailored, optimal therapies can begin sooner – when time matters most.
Product is FDA cleared. Availability of product in each country depends on local regulatory marketing authorization status.


The MIC and SIR data you need, when you need it.
Every ASTar report is comprehensive and easy to interpret, containing:
- Truly accurate Minimum Inhibitory Concentration (MIC) values
- and SIR categorization
So you know exactly which antibiotic and dosage to use.
With LIS compatibility, ASTar integrates into your hospital routines and seamlessly delivers AST results from bench to bedside to help guide treatment decisions.

Get in touch
We would love to talk
Tailored medicine.
The ASTar panel covers a range of bug/drug combinations to ensure on-scale actionable results are obtained from a single run. Stay one step ahead of bacteria and give the appropriate treatment sooner.
The disc is designed to facilitate future panel expansion with new antimicrobials.
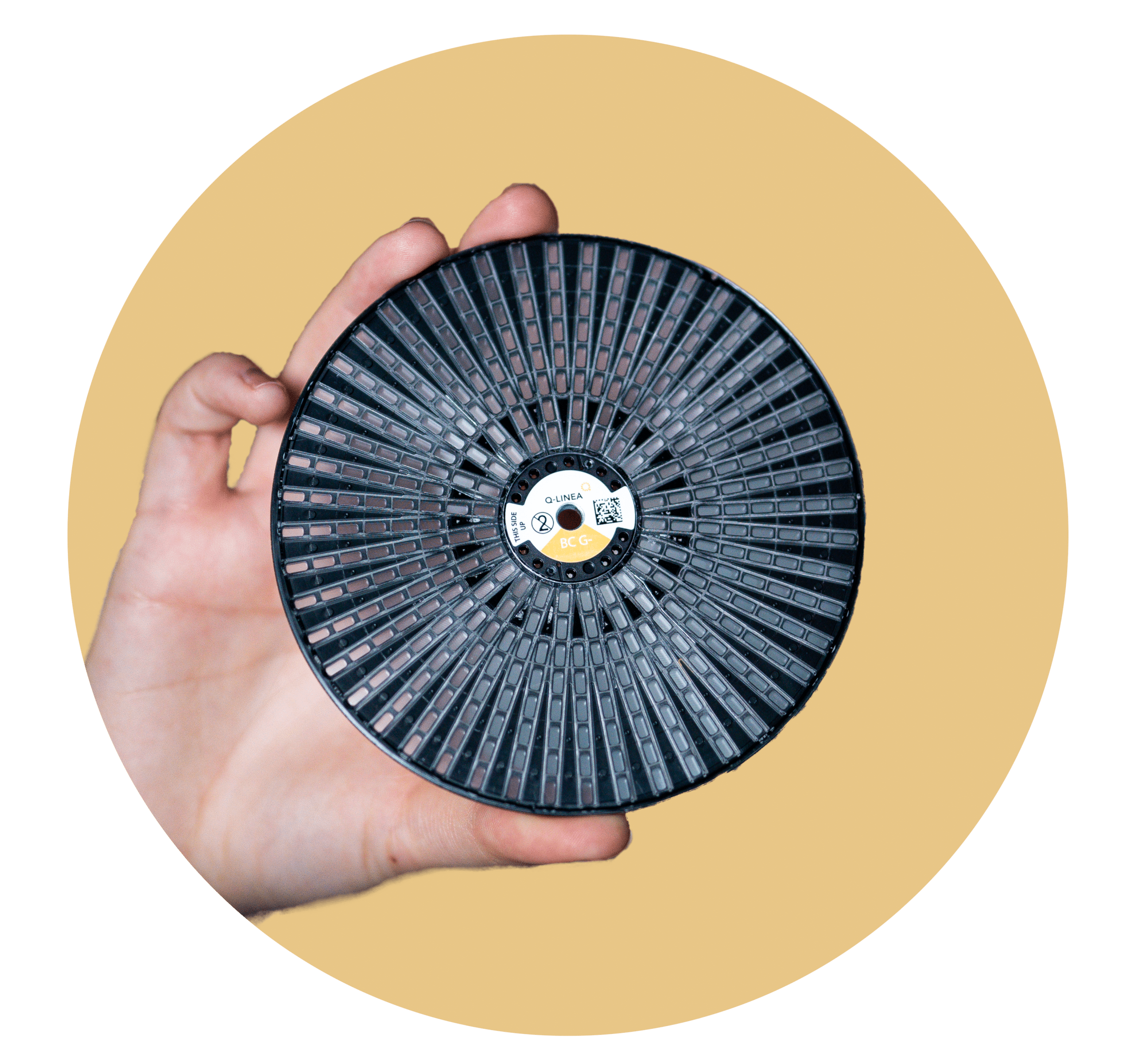

A new member of your stewardship team.
Rapid AST helps expedite treatment decisions and could reduce patient exposure to unnecessary antimicrobials, mitigating the emergence of antimicrobial resistance (AMR). Support infection prevention and control by detecting multidrug-resistant organisms (MDROs) sooner.
As the threat of AMR and challenging infections continues to rise, rapid AST is a strong addition to your antimicrobial stewardship programs.
Future-proof.
The ASTar software is kept up to date using the latest regulatory breakpoints, ensuring that ASTar is an invaluable tool in the ever-evolving world of antimicrobial treatments and antimicrobial resistance.
We handle breakpoint updates, so you don’t have to.

Experience the difference
Product is US FDA 510(k) cleared.
Availability of product in each country depends on local regulatory marketing authorization status.
“A huge advantage”
Listen to Ehsan Ghaderi, Head of Department, and Sofia Persson, Consultant Physician, Bacteriology Uppsala University Hospital, talk about how ASTar have helped save time in the lab.“We need to implement fully automated systems”
Jan Gorm Lisby, Chief Consultant, Amager and Hvidovre Hospital, Department of Clinical Microbiology, talks about the challenges facing laboratories today and the need for automated solutions.
Looking for our posters and publications?
Click here

Contact our US team
We’re here for you and ready to support you. Contact us if you have any questions, or want to get a demo or a service visit.
The story of Q-linea
Back in 2008, three people crammed into an office the size of a cleaning closet were working on an idea. That idea led to a international team, working all over the world with a single focus – to improve sepsis diagnostics.
How can ASTar help in the lab?
Read more
